
Company
Engineering Inside
ENSIDE is a digital healthcare company expanding
the boundaries of next-generation medical device technologies
Leveraging Physical AI and Agentic AI technologies, we develop implantable and wearable biomedical hardware,
leading the commercialization of medical devices with regulatory approval and GMP compliance.
At the intersection of technology, clinical practice, and industrialization, ENSIDE is redefining the paradigm of healthcare.
Key Business Areas
Physical
AI
Agentic AI
Technology
-
We are developing an intelligent therapeutic interface that detects and processes biosignals in real time, autonomously making decisions and interventions based on the situation.
-
The AI algorithm acts as an intelligent agent, capable of performing medical interventions directly in and around the body, beyond mere data interpretation.
-
Development of Implantable and Wearable Medical Devices
-
We are developing implantable devices based on ultra-thin, flexible electronic components that can be applied to both the central and peripheral nervous systems, while also providing non-invasive diagnostic and therapeutic solutions through wearable and skin patch devices.
-

Regulatory Approval and GMP System of Medical Devices
-
Based on comprehensive regulatory experience with global standards such as KMFDS, FDA, and CE, we provide end-to-end solutions covering Quality by Design (QbD), clinical trials (IND/IDE), and large-scale manufacturing through GMP-compliant facilities.
ENSIDE’s Differentiators
- Integration of AI and Bioelectronics
- AI Hardware Platform Enabling Physical Intervention
- One-Stop Medical Device Solutions: From R&D to Commercialization
- Planning Development Regulatory Approval Manufacturing Clinical Trials / Commercial Launch
- Multidisciplinary Team Structure
- An integrated collaborative framework of experts in medicine, mechanical and electronic engineering, artificial intelligence, and regulatory affairs.
Our Vision
Next-gen Agentic Medical Devices Autonomously sensing, deciding, and acting both inside and outside the body

Revolutionizing Diagnosis and Treatment Paradigms for Intractable Diseases, Including Total Paralysis, Neurological Disorders, and Cardiovascular Conditions
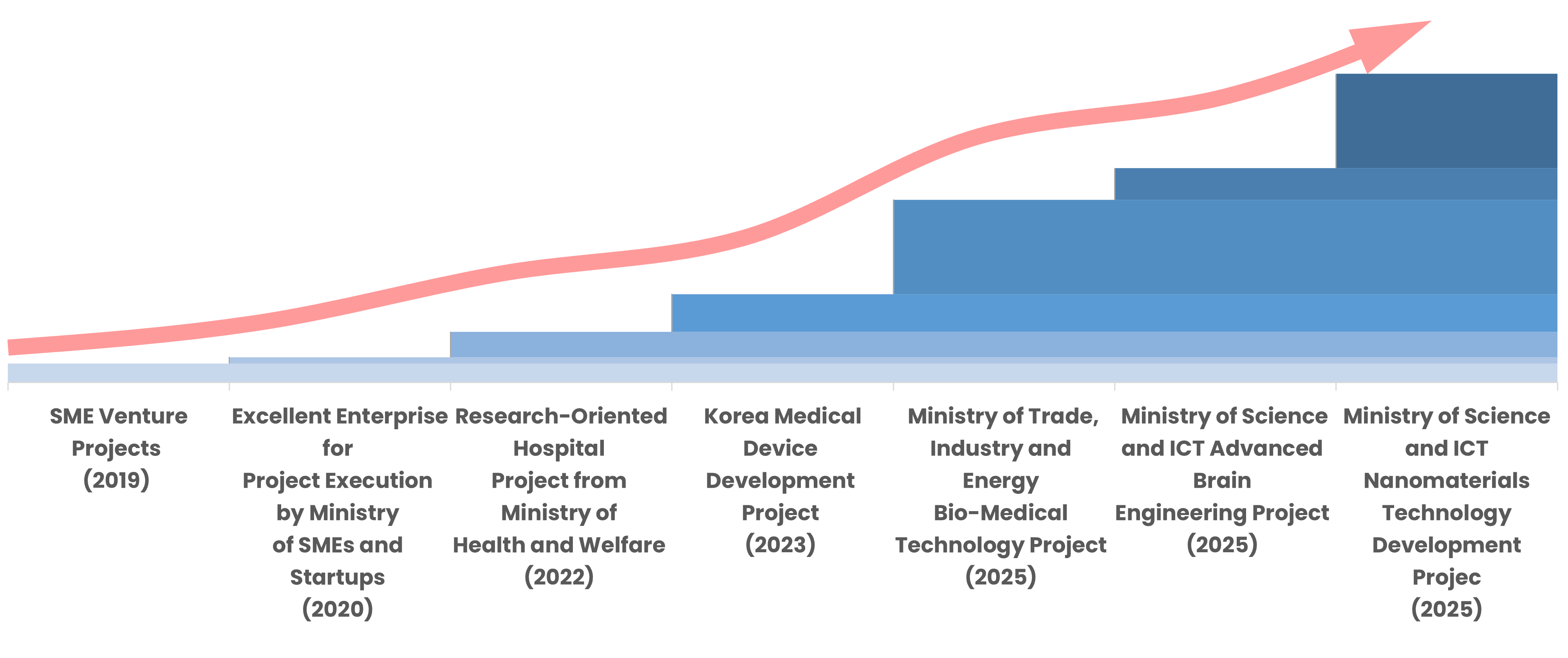
Company Milestones
-
2025
- Ministry of Trade, Industry and Energy Bio-Medical Technology Project
- Ministry of Science and ICT Advanced Brain Engineering Project
- Ministry of Science and ICT Nanomaterials Technology Development Project
- GMP-compliant manufacturing setup
-
2024
- Ministerial Award from Ministry of Science and ICT
-
2023
- Korea Medical Device Development Project
- MOU with SK Telecom, Yonsei University, Sevrance Hospital
- Specialized Research Company
-
2022
- Research-Oriented Hospital Project from Ministry of Health and Welfare
-
2021
- Supplied clinical research products to domestic tertiary hospitals
-
2020
- Company-Affiliated Research Institute
- Excellent Enterprise for Project Execution by Ministry of SMEs and Startups
-
2019
- SME Venture Projects
- Founded Firm as Employee Startup at Daegu Gyeongbuk Institute of Science and Technology (DGIST)
Company Milestones
Intellectual Property
-





Patent
-

Data Transfer Agreement
-

Ministerial Award


